Genome-wide association study on coronary artery disease in type 1 diabetes suggests beta-defensin 127 as a risk locus
We have extensively researched CAD genetics in T1D. Our findings suggest many novel genomic regions including a region near β-defensin 127 that provides a link between diabetes, infection susceptibility, and CAD.

Diabetes is a common disease affecting 422 million people worldwide – half a million in Finland – and the incidence is steadily rising. Type 1 diabetes (T1D) is an autoimmune form of insulin deficiency, mainly diagnosed in childhood or young adults. Despite insulin and other diabetes treatments, the elevated blood glucose levels damage blood vessels leading to microvascular complications affecting for instance the kidneys, and to macrovascular complications including coronary artery disease (CAD) – a severe complication that may lead to death. In fact, it has been estimated that the risk of CAD is 10 times higher in individuals with T1D compared to those without, and there is accumulating evidence that the pathogenesis of CAD differs in T1D. CAD is not only caused by poor life-style habits: The heritability of CAD has been estimated roughly 40% in the general population, and to date, 163 genomic regions has been associated with CAD, of which one even led to a new medicine (PCSK9 inhibitors). Despite the need, the genetic background of CAD has not been extensively studied in T1D.
The Finnish Diabetic Nephropathy (FinnDiane) study is a nationwide study aiming to identify clinical, biochemical, environmental and genetic risk factors for diabetic complications among participants with T1D. We performed a genome-wide association study for CAD in 4869 Finnish individuals with T1D of whom 941 had experienced a CAD event. A genome-wide association study explores millions of genetic markers for each participant to scan the entire human genome for potential genetic regions and genes associated with the studied disease.
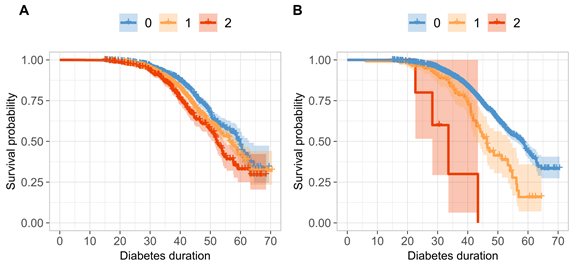
Proportion of participants without CAD (survival probability) according to number of risk-increasing variants they carry (0, 1, 2): A. CDKN2B-AS1, B. DEFB127.
We discovered for the first time the well-known genomic risk-region, CDKN2B-AS1,robustly associated with CAD also in individuals with T1D. Risk-increasing variants on CDKN2B-AS1 are known to cause many diseases such as cancer, diabetes, and CAD – now also CAD in T1D. In addition, we found a completely novel genomic region near DEFB127, a gene coding for β-defensin 127, although still needing an external validation. β-defensins are antimicrobial proteins, which help our bodies fight against bacterial infections and specific types of viruses. Importantly, infection susceptibility has previously been linked to CAD in people with T1D. We also discovered three putative genomic regions, which we linked to specific cardiovascular measures; central blood pressure (B3GNT2), intima media thickness (CNTNAP5), and serum leucocyte levels (GRAMD2B).
Another important aspect in genome-wide studies is the predictive power hidden in our genomes for the diseases we are likely to develop. We calculated a genetic risk score with the known genomic CAD risk-regions and demonstrated an association with CAD also in T1D. Genetic risk scores could be used in the clinics for identifying high-risk patients before they develop symptoms in order to introduce them preventative treatments.
We have extensively researched CAD genetics in T1D. Our findings suggest many novel genomic regions including a region near β-defensin 127 that provides a link between diabetes, infection susceptibility, and CAD.
Original article:
Genome-wide association study on coronary artery disease in type 1 diabetes suggests beta-defensin 127 as a risk locus. Antikainen AAV, Sandholm N, Trégouët DA, Charmet R, McKnight AJ, Ahluwalia TS, Syreeni A, Valo E, Forsblom C, Gordin D, Harjutsalo V, Hadjadj S, Maxwell AP, Rossing P, Groop PH. Cardiovasc Res. 2021 Jan 21;117(2):600-612. doi: 10.1093/cvr/cvaa045. PMID: 32077919 Free PMC article.
