Cystatin B-deficiency triggers ectopic histone H3 tail cleavage during neurogenesis
This study indicates that CSTB-deficiency causes defects in epigenetic gene regulation during neurogenesis. These findings constitute the earliest molecular changes reported in Cstb−/− mice, implying a key role for epigenetic alterations in triggering the pathogenesis associated with CSTB deficiency. They provide a likely explanation for the neurodevelopmental defects linked to a complete loss of CSTB function, and open new avenues for the development of novel therapies for EPM1.

Unverricht-Lundborg type (EPM1) is an inherited neurodegenerative disorder and the most common form of progressive myoclonus epilepsy in the world. EPM1 patients develop neurological symptoms at 6-16 years of age. These are highly disabling and resistant to treatment, urging development of disease-specific therapies. Increased understanding of molecular mechanisms underlying EPM1 is essential for development of new therapeutic strategies for the benefit of EPM1 patients.
EPM1 is caused by biallelic loss-of-function mutations in the CSTB gene, leading to reduced expression of the cystatin B protein (CSTB). CSTB mutations resulting in a complete loss of CSTB expression cause a more severe brain disease, presenting with neurological defects during embryonic development. The genotype-phenotype correlations in patients with biallelic CSTB mutations predict a pivotal role for CSTB in regulating brain development and maturation.
CSTB has been characterized in detail as an inhibitor of cysteine proteases. However, its physiological function is unknown. In the present study, the molecular function of CSTB during brain development was investigated in an in vitro model of neural stem cell renewal and differentiation derived from embryonic mouse brains.
In differentiating wild-type (wt) neural stem cells, cysteine proteases cathepsin B and L were shown to cleave the N-terminal tail of histone H3, a protein domain with important implications in epigenetic regulation of gene expression and chromatin structure. This molecular signature was mostly detected in a subset of new-born neurons (Ki-67-negative doublecortin-positive cells), suggesting that histone H3 tail cleavage is particularly involved in the process of neurogenesis. Neural stem cells and early differentiating neurons derived from CSTB-deficient (Cstb-/-) mice displayed drastically elevated levels of cleaved histone H3. Reincorporating CSTB into CSTB-deficient cells or treating these cells with molecules inhibiting specific protease activities abolished histone H3 tail proteolysis, indicating that CSTB acts as an endogenous inhibitor of the cysteine proteases responsible for histone tail cleavage in the neural cell lineage.
In the absence of CSTB, neural stem cells preserved their ability to generate neurons and glia. However, they displayed reduced self-renewing capacity and increased differentiation rate toward an astrocyte fate. These observations indicate that CSTB deficiency leads to altered neural stem cell dynamics. Finally, using high-throughput transcriptome profiling (RNA-seq), subtle, yet significant gene expression changes in neural cells lacking CSTB were detected, highlighting altered expression of nuclear-encoded mitochondrial genes. If was further confirmed that neural stem cells lacking CSTB acquired mitochondrial respiratory defects upon induction of differentiation.
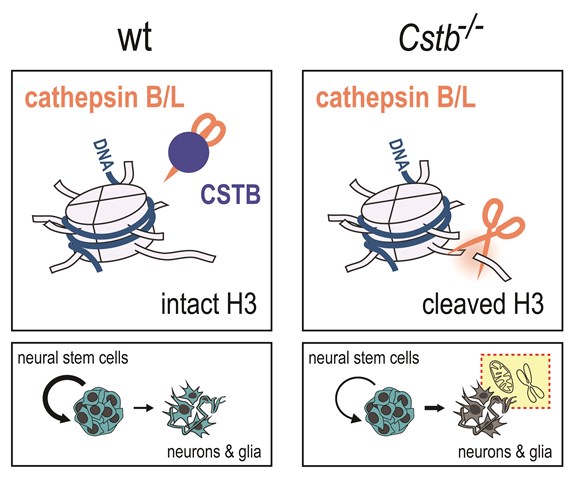
The data indicate that CSTB-deficiency causes defects in epigenetic gene regulation during neurogenesis. These findings constitute the earliest molecular changes reported in Cstb−/− mice, implying a key role for epigenetic alterations in triggering the pathogenesis associated with CSTB deficiency. They provide a likely explanation for the neurodevelopmental defects linked to a complete loss of CSTB function, and open new avenues for the development of novel therapies for EPM1.
Original article:
Cystatin B-deficiency triggers ectopic histone H3 tail cleavage during neurogenesis. Daura E, Tegelberg S, Yoshihara M, Jackson C, Simonetti F, Aksentjeff K, Ezer S, Hakala P, Katayama S, Kere J, Lehesjoki A-E, Joensuu T. Neurobiol Dis. 2021 Aug;156:105418. doi: 10.1016/j.nbd.2021.105418.
