Molecular genetics research on a group of rare genetic diseases called the Finnish Disease Heritage has been a key focus of The Folkhälsan Research Center for decades. A new review article published in Disease Models and Mechanisms gives a fresh update on this interesting and evolving group of diseases.
It is important to continue studies with rare diseases, which often help advancing understanding of more common disorders with similar symptomatic repertoire.
Hereditary monogenic disorders are caused by single, usually autosomal-recessive gene variants and can be completely sporadic or enriched in historically isolated populations due to past genetic drift events. In Finland, distinct subpopulations have remained and maintained their unique genetic repertoire, partially due to relative geographic isolation and several evolutionary bottlenecks leading to social isolation on a population level. The Finnish disease heritage is a prominent example of this phenomena. In Finland about one in five persons carries a gene variant associated with at least one Finnish heritage disease, and about one in 500 children born is affected.
This is partially due to the prominence of what is called population isolates. Population isolates are defined as subpopulations originally derived from a small group of individuals who became isolated because of a certain founding event, e.g. settlement of a new territory, and have remained isolated for several generations. This is called the founder effect, and is an example of a bottleneck phenomenon in which, for one reason or another, only a few survivors of a population remain to repopulate. Other typical evolutionary bottlenecks are famine, epidemics and war. Finland’s population history is a great example of this, being characterized by the founder effect, several bottlenecks, isolation and the subsequent genetic drift.
In the early 1960’s, Dr Reijo Norio conducted genealogical studies on congenital nephrosis, an inherited disorder characterized by protein in the urine and swelling of the body, often resulting in infection and kidney failure. He discovered the autosomal-recessive mode of inheritance of this disease. An autosomal-recessive genetic condition must be passed down from both parents in order to manifest, become phenotype. In contrast, autosomal-dominant inheritance only requires getting the gene defect from one parent. In the wake of this discovery, Dr. Norio later described how the founder effect and isolation had nudged the Finnish gene pool towards both having over- and under representation of distinct genetic diseases.
Enter the Finnish disease heritage, the FDH. An FDH disease is a genetic disease or disorder that is significantly more common among the Finnish than other populations. Finnish physicians were often the first to identify and describe many of these diseases, however, their foreign colleagues have later identified some of the diseases in other population isolates as well.
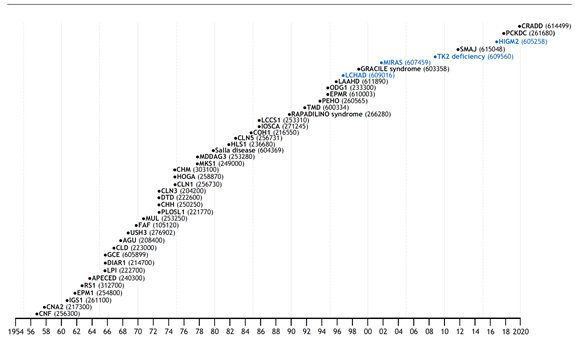
"The Perheentupa-steps" (Uusimaa et al. 2022). This widely used traditional representation of the Finnish disease heritage (FDH) diseases organizes them chronologically based on the year in which the disease description was published. The respective Online Mendelian Inheritance in Man (OMIM) codes are in parentheses. FDH diseases are in black and candidate diseases in blue.
As research has progressed, geneticists can now relatively easily pinpoint whether a new FDH candidate disease has a founder mutation and whether the allele frequency clearly differs between Finnish and other populations in the world. Today, the FDH contains 39 diseases in total. 34 of them autosomal-recessive, three autosomal-dominant, and two X-linked (meaning that the genes are only found on X-chromosomes). The Folkhälsan Research Center (FHRC) has contributed to around half of these findings.
The FDH has opened the door for genotype-phenotype discoveries, pioneering genetic research and, more recently, to research into the underlying molecular mechanisms and potential treatments. But the FDH itself is evolving, too. In the new review article, published in Disease Models and Mechanisms, a group of expert authors including Dr. Jukka Kallijärvi working on GRACILE-syndrome at the FHRC, give a fresh update on the FDH.
– Prof. emerita Vineta Fellman’s decades-long and my more recent work on GRACILE syndrome is an example of a successful trajectory from clinical work via genetics to experimental research aiming at understanding the molecular disease mechanism and developing treatments, says Jukka Kallijärvi.

Docent Jukka Kallijärvi is studying the GRACILE-syndrome at the FHRC.
Science evolves progressively and research seldom yields immediate results, but the hopes are high, and the effort is deemed to be worth it in the long run.
– For many of the FDH diseases the experimental work is still awaiting to be started. It is a demanding and expensive endeavor and not all diseases can be easily studied in experimental models such as cell cultures or mice, but this should not discourage us from doing it, Kallijärvi notes.
Even though the benefits of studying rare diseases might seem marginal, advancing the frontier within genetic science has a habit of giving new insights and deepening understanding of human genetics in general. As such, results tend to end up benefiting medicine as a whole.
– It is important to continue studies with rare diseases, which often help advancing understanding of more common disorders with similar symptomatic repertoire. Utilization of modern methodology in experiments tackling the FDH provides missing information of the physiological functions that the disease-causing genes carry out under physiological conditions. For this, we are creating new in vivo models with CRISPR/Cas9 genome editing to generate preclinical models that enable novel treatment discoveries for these devastating diseases, adds Dr. Satu Kuure, one of the corresponding authors of the review.
The researchers underline that maintaining the ongoing research to identify novel disease-causing variations and comprehend their molecular and cellular pathomechanisms is crucial. These combined efforts will make it easier to diagnose uncommon diseases and lay the foundation for a better knowledge of more common diseases in the future.
Read the whole study here.
Simon Granroth, Science Communicator
Reference:
Uusimaa J, Kettunen J, Varilo T, Järvelä I, Kallijärvi J, Kääriäinen H, Laine M, Lapatto R, Myllynen P, Niinikoski H, Rahikkala E, Suomalainen A, Tikkanen R, Tyynismaa H, Vieira P, Zarybnicky T, Sipilä P, Kuure S, Hinttala R. The Finnish genetic heritage in 2022 - from diagnosis to translational research. Dis Model Mech. 2022 Oct 1;15(10):dmm049490. doi: 10.1242/dmm.049490. Epub 2022 Oct 26. PMID: 36285626; PMCID: PMC9637267
